Electroplating.net
Your Guide To Electroplating On The Net
Silver Plating, Gold Plating, Chrome Plating, Rhodium Plating...
Electroplating Process
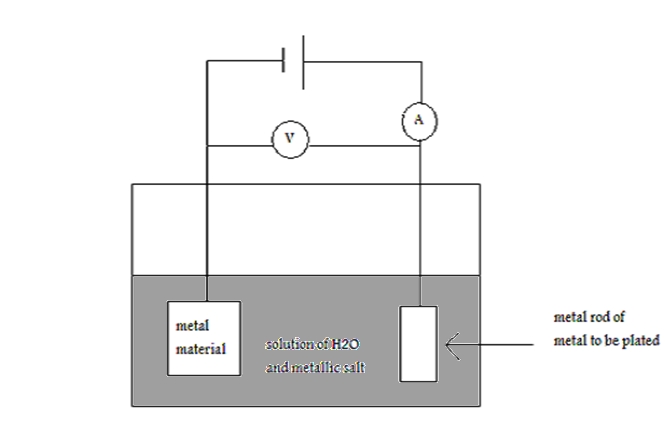
What is electroplating? Electroplating, also known as electrodeposition, occurs when electric current is used to coat the surface of a metal material. This coating is produced by using the metal material to be coated as a cathode immersed in a metallic salt in an electrolytic cell. The metal of the salt is the metal used to form the plate on the metal material. What is the electroplating process? Before a metal can undergo the process of electroplating, the metal to be plated must first be identified and properly cleaned. The cleaning done to the metal is only a cleaning performed for the purpose of removing dirt, oil, and other debris from the top layer of the metal material. The cleaning should not change the properties of the metal material. Only the color of the metal material should change as the surface is now dirt-free so that no obstructions will be present to prevent the bonding of the plate onto the metal material. After every step, the metal must be rinsed in order to remove chemicals that may remain from previous steps. This rinsing will prevent cross-contamination. Then the metal should be descaled with the appropriate acid descaler. After rinsing, the metal, if required, should be pre-plated and rinsed. Often times this pre-plated step is required where an intermediate layer is added between the metal material and the desired plate to be added. This intermediate layer is often required in order to slow down or stop reactions that occur between the metal material and the metal plate added. The next step is final plating where electroplating occurs. The “plating bath” known best as the electrolyte, is prepared with aqueous salt containing positively charged ions of the metal to be plated on the metal material. The metal material to be plated is immersed in the aqueous solution of the electrolyte, usually in the middle, and is attached to the negative end of the power source making the metal material the negatively charged cathode of the electrolyte. The anode of the electrolyte is a metal rod of the metal to be plated on the metal material. This rod is attached to positive end of the power supply and is placed at the edge of the electrolyte. With the power source supplying the predetermined appropriate amount of circuit, the electroplating process can begin. The positively charged anode causes repulsion between the rod and the positive metal ions in the aqueous solution. At the same time the negatively charged metal material cathode causes an attraction between the metal material and the positively charged metal ions in the aqueous solution. Thus, the metal ions in solution migrate to the negatively charged metal material and through the process of electrolysis the metal ions are removed from the aqueous solution and placed onto the surface of the metal material. After this step is completed the metal material is rinse and post treatments are preformed on the metal material to ensure the plate surface is maintained. Once again the metal material is rinsed and finally dried and packaged, ready for sell.
See Electroplating in action on the following video.
Why is typical thickness of layers? The thickness of the layers of the plate is determined by the shape of the metal material. If the metal material is characterized by more sharp features, then deposition is likely to be more dominate at the sharp features/ edges, due to the direction of the electric field lines between the anode and cathode of the process. In general, however, the layer of the plating has a thickness from 0.1 to 30 microns. In maintaining uniformity of layering when using electroplating, the placement of the anodes in the electrolyte is highly important. What are the applications of electroplating? Electroplating is used on silverware, jewelry, automobiles, motorcycles, hand and medical tools, light fixtures, steel bolts, nuts, and washers, electrical connectors, and in many industries. Silverware will resist wear longer when electroplated. Automobiles and motorcycles have chrome plating on their steel parts. Chrome plating is added to the steel parts of many hand tools and medical supplies in order to keep the tools from wearing. Zinc is added to steel bolts, nuts and washers. And in many industries electroplating is used for better functioning of the machinery, to prevent corrosion of the metals and for decoration. What are some issues with wastes from electroplating? Wastewater is formed in each of the process baths of the electroplating process where rinsing is performed and the level of danger associated with the wastewater depends on the level of toxic solutions and metals used to perform the electroplating. Spillage and dumping of the process bath water can further contribute to the issues of waste with electroplating. When cleaning process tanks themselves, large amounts of waste containing toxic organics and metals can be produced. Hot plating baths form solvents and vapors that can elevate levels of volatile organic compounds (VOCs) and volatile metal compounds. Furthermore, the mixing of cyanide and acidic wastewaters should be avoided as this mixture produces lethal hydrogen cyanide gas. What were past dangers of electroplating? The lack of proper ventilation systems in industry in the past has lead to many health hazarders for industry workers. Fumes created from this process are dangerous to breathe in. Extremely dangerous chemicals are sometimes used in electroplating that were highly misunderstood in the past. Cyanide compounds are highly desired as cyanide will remove any undesired impurities and will caused an even layer of metal deposit with low levels of sensitivity towards impurities to form on the metal material. At the same time, cyanide exposure obstructs the proper functioning of the metabolic system and caused rapid death. Chromium is a popularly used metal compound in electroplating as it a great corrosion-resist surface while also having a bright and attractive appearance. One of the chromium compounds used in electroplating, hexavalent chromium, is highly toxic and can cause ulcers in form in the nasal membrane, and is believed to cause lung cancer when overexposure occurs. |
Electroplating Info Electroplating Process Silver Plating Gold Plating Chrome Plating Home Electroplating Kits Contact Us
Electroplating Guide On The Net
© Copyright Electroplating.net, 2010. All Rights
Reserved.
www.electroplating.net